Sodium bicarbonate or Sodium hydrogen carbonate has a monoclinic crystalline structure. Nicolas Leblanc a French chemist produced sodium carbonate in the year 1791. In the year 1846 Austin Church and John Dwight, bakers of New York started the first factory to produce baking soda. It is a white solid crystalline chemical compound usually in its powder form. This salt is composed of sodium ions and bicarbonate ions. Its molecular formula is NaHCO3. It is a weak base. It is commonly called as baking soda and is used in cooking. PH value is about 8.31.
| Sodium bicarbonate Chemical formula | NaHCO3 |
| Molecular Weight/ Molar Mass | 84.0066 g/ mol |
| Density | Solids: 2.20 g/cm3 |
A few other important properties of sodium hydrogen carbonate are listed below.
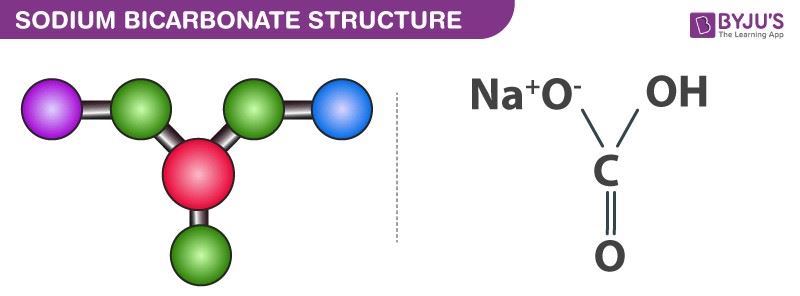
Sodium bicarbonate molecules feature one sodium cation and one bicarbonate anion. Here, an ionic bond is formed between the positively charged sodium ion and the negatively charged oxygen (which is singly bonded to the central carbon and not bonded to a hydrogen atom).

Learn more about the chemical behaviour and importance of Sodium bicarbonate with the expert faculties at BYJU’S.
Test Your Knowledge On Nahco3!