States of Matter: Solid, Liquid, and Gas

In chemistry, the behavior of molecules determines the states of matter, or phase, of a substance. The states of matter describes how molecules move, behave, and organize themselves in space. Before delving into a state of matter, it’s important to understand the fundamental definitions of matter and its constituents. It turns out that matter includes everything on Earth. Anything with mass and physical space is matter. There are countless shapes and forms that matter can take, and it is made up of numerous different substances known as elements.
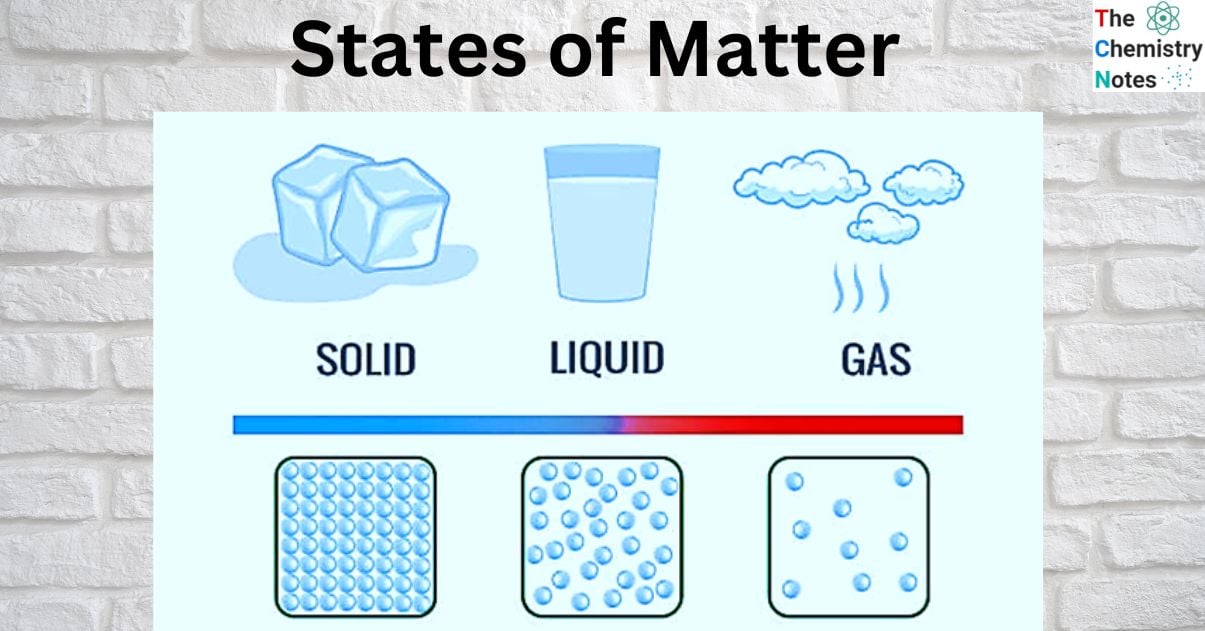
Table of Contents
Interesting Science Videos
States of Matter: Solid, Liquid, and Gas
In most environments, matter can exist as a solid, a liquid, or a gas, which are the three distinct physical states of matter. Other states, such as plasma, Bose-Einstein condensates, and neutron stars, may exist in extreme environments. It is thought that there may be additional states, such as quark-gluon plasmas. In the form of dense stars and rarefied interstellar medium, hot plasma makes up a large portion of the universe’s atomic matter. However, we will discuss about solids, liquids, and gas in this topic.
Solids, liquids, and gases are classified as different states of matter because their atoms and molecules are organized differently. Solid molecules are densely packed, whereas gas molecules move freely. This molecule organization is significant because it provides each state of matter with its own set of distinct properties. Tightly packed solids are frequently hard, whereas gases have no defined shape due to their freely moving molecules.
Solid State
In a solid, the particles are arranged, closely clustered, and don’t move or mix. Solids have their own shape due to their fixed positions. One factor that affects how atoms or molecules are arranged in a solid is the size of the substance that they are made of. The sizes of the atoms in various elements differ; some are smaller or larger than others. Atoms of various sizes can form unusual structures when they are packed closely together. For instance, the size of the atoms in a molecule of table salt (NaCl) varies. Because the sodium (Na) atoms are smaller than the larger chlorine (Cl) atoms, they are sandwiched between the latter.
Properties of Solid State
- Solids have a distinct volume and shape. They typically have definite density.
- A solid’s molecules are held together by strong cohesive forces (inter-particle forces).
- A solid’s molecules are fixed at one point. Thus, intermolecular forces of attraction keep them firmly in place. Solids cannot therefore be poured. There is very little solids diffusion.
- Every pure solid has a unique melting point that is determined by the forces of intermolecular attraction. At atmospheric pressure, it is warmer than the ambient temperature.
- The majority of solids are rigid, hard, and incompressible.
- At a specific temperature, the vapor pressure of a solid is significantly lower than that of a liquid.
Liquid State
Although atoms in a liquid are semi-organizedly arranged in a defined space, they are still free to move and occasionally mix in unexpected ways. In contrast to solids, liquids do not have a fixed structure or a tight bond between their atoms. But just like a solid, the interactions between atoms are what give a liquid its characteristic properties. Liquids are easily able to mix, spill, and change shape. The third distinguishing characteristic of liquids is their capacity to change shape, which is governed by the same forces that hold them together.
In a liquid, molecules are held together by intermolecular forces that are typically weaker than chemical bonds. These forces enable molecules to adhere to one another while still allowing for mixing and movement. Atoms are held together in liquids by three different intermolecular forces: hydrogen bonds, dipole-dipole forces, and dispersion forces.
Properties of Liquid
- Liquids always have a fixed volume. Unless they are impacted by outside forces, they always have the same volume no matter what.
- In a liquid, pressure is more or less constant. Practically speaking, liquids cannot be compressed
- When pressure is applied to a liquid, the force is dispersed throughout the substance. ‘Pascal’s Law’ has been used to explain this phenomenon.
- A liquid’s surface is very malleable. Liquids also possess a special characteristic known as surface tension. A liquid can withstand the impact of invading external forces if it is cohesive enough.
- Different liquids have different densities. The densest liquids, though, still have a lower density than solids.
- Liquids always move from a higher to a lower plane. Rivers, for instance, always flow downward
- Liquid molecules are held together by electrical attraction.
- The molecules or particles of liquids are joined together due to their electrical attraction to one another. They are therefore frequently very conductive as a result. For instance, one of the best conductors of electricity is the frequently encountered substance water. In contrast, the electrical conductivity of pure water that has not been tainted by metals or other elements is low.
- Liquids do not have particularly high thermal conductivities by nature. Thermal conductivity and thermal energy conductivity are not the same. Some liquids conduct heat more slowly than others. Different liquids have a higher level of thermal conductivity.
Gaseous State
Gas particles are dispersed widely, move quickly, and lack any particular organization. The atoms and molecules in gases are not particularly attracted to one another, in contrast to the particles in solids and liquids.
The intermolecular forces that hold molecules in liquids and some solids together are still present in gases, but gas molecules are able to break free from them quickly. Since gas molecules have a lot of energy, they are constantly moving and are never still.
Because of this, atoms or molecules in a gas regularly pass one another and only occasionally interact. The most distinctive characteristics of gases are determined by this absence of force holding atoms or molecules together. A gas will spread out to take on the shape of its container and will spread out to fill any space. The forces that exist between atoms or molecules are less significant in this state of matter. The movements of atoms or molecules in a gas are instead governed by three additional factors: temperature, pressure, and volume.
Properties of Gases
- Gases are less dense than solids or liquids because they contain scattered molecules that are dispersed throughout a given volume. Gas particles can move quickly and randomly past one another due to the fluidity that their low density confers, expanding or contracting without maintaining a fixed position. Since the average distances between the molecules are so great, molecular interactions don’t affect how the molecules move.
- Gases lack a distinct volume or shape. Gas molecules can expand or contract to fill the volume of the container they are held in due to their random movement. The space of the container in which a gas’s molecules have room to move is referred to as the gas’s volume as a result. Due to this characteristic, gases take up more space than they would in a liquid or solid state. As temperature and pressure change, gases will also expand and contract in predictable ways.
- Gases can be compressed because of their low density, which allows their molecules to be spaced widely apart. They can now move around freely and squeeze into the spaces between one another. Gases have both the ability to expand and compress. The freedom of gas molecules causes them to conform to the shape and volume of any container they are placed in.
- Due to the substantial distances between gas molecules, it is possible for two or more gases to quickly and easily combine to create a homogeneous mixture. Dispersion is the name of this process.
- The molecules of a gas are constantly moving. On the interior surface of their container, they apply pressure, or force per unit area. The volume of gas contained in a particular container, the pressure, and the temperature all affect the pressure differently.
References
- Solid State Physics: Structure and Properties of Materials. Alpha Science. pp. 1–3. ISBN 978-1-84265-218-3.
- Goodstein, D.L. (1985). States of Matter. Dover Phoenix. ISBN 978-0-486-49506-4.
- Wahab, M.A. (2005). Solid State Physics: Structure and Properties of Materials. Alpha Science. ISBN 978-1-84265-218-3.
About Author



